Topic 1
1) What is an atom?
Answer 12) Describe the structure of an atom.
Answer 23) What are the relative charges and masses of protons, neutrons and electrons?
Answer 3Protons: mass 1, charge +1
Neutrons: mass 1, charge 0
Electrons: mass almost zero, charge -1.
4) Why do atoms contain the same number of protons and electrons?
Answer 45) How would you describe the size of the nucleus relative to the rest of the atom?
Answer 56) Where is most of the mass of the atom found?
Answer 67)What is the mass number of an element?
Answer 78) What is the atomic number of an element?
Answer 89)The number of which particle is unique to an element and gives it its identity?
Answer 910) If an atom contains 12 protons, how many electrons will it have?
Answer 1011) If an atom has a mass number of 23 and an atomic number of 11, how many protons, neutrons and electrons does it contain?
Answer 1111 electrons
23-11 = 12 neutrons
12) What is an isotope?
Answer 1213) What is the relative atomic mass, (Ar)?
Answer 1314) Why do some elements have a relative atomic mass that is not a whole number.
Answer 1415)What is the formula for calculating relative atomic mass of an element from the relative mass and abundance of its isotopes?
Answer 15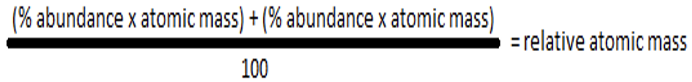
16) How did Mendeleev arrange the elements known at the time into a periodic table?
Answer 1617) How did Mendeleev use his table?
Answer 1718)Why does Mendeleev’s method of organising elements in order of increasing atomic mass not always work?
Answer 1819) How are elements in the modern periodic table arranged?
Answer 1920) Where are the non-metals found in the periodic table?
Answer 2021) What do all elements in the same row of the periodic table have in common?
Answer 2122)What do all elements in the same column of the periodic table have in common?
Answer 2223)What is an ion?
Answer 2324) Describe how an ionic bond is formed.
Answer 2425)Is a cation positively or negatively charged?
Answer 2526) Is an anion positively or negatively charged?
Answer 2627)What charge do the ions have when formed from elements in group:
- 1
- 2
- 6
- 7
- +
- 2+
- 2-
- –
28)
What do the compound endings:
- ide
- ate
mean?
Answer 28- ide – a compound of only the named substances
- ate – a compound of the named substances and oxygen
29) What is the formula of the compounds formed from:
- Mg2+ and Cl–
- Na+ and O2-?
- MgCl2
- Na2O
30)Describe the structure of ionic substances.
Answer 3031)How many electrons does Mg2+ have? Mg has an atomic number of 12
Answer 3132) Describe what happens in covalent bonding?
Answer 3233)What does covalent bonding result in the formation of?
Answer 3334) Name and explain two physical properties of ionic compounds.
Answer 34- They have high melting and boiling points because there are strong electrostatic forces holding the oppositely charged ions in place, therefore a lot of energy is needed to separate the ions.
- They can conduct electricity when molten or in aqueous solution (dissolved in water) because the ions are free to move and carry their charge.
35)Name and explain two physical properties of covalent, simple molecular compounds.
Answer 35- They have low melting and boiling points because there are weak intermolecular forces of attraction between molecules.
- They do not conduct electricity because the molecules are not charged.
36)
Describe the structures of:
- Diamond
- Graphite
- Each carbon atom is held in place by 4 strong covalent bonds to other carbon atoms. This arrangement is replicated throughout the whole structure creating a giant structure.
- Each carbon atom is held in place by 3 strong covalent bonds. This creates flat layers of carbon atoms which stack on top of each other. The unused outer electron on each carbon atom sits between these layers and is delocalised (free to move).
37) Why is diamond used in cutting tools?
Answer 3738) Why does diamond have such a high melting point?
Answer 3839) Why does graphite conduct electricity?
Answer 3940) Why can graphite act as a lubricant?
Answer 4041)What are fullerenes? Explain its properties in terms of its structure and bonding.
Answer 4142) What is graphene? Explain its properties in terms of its structure and bonding.
Answer 4243) Describe polythene’s structure
Answer 4344) Describe the bonding in metals?
Answer 4445) Why do metals conduct electricity?
Answer 4546) Why are metals malleable?
Answer 4647) Why is it difficult to represent models of compounds on paper?
Answer 4748) What are the properties of most metals?
Answer 4849) What is an empirical formula?
Answer 4950) What is the law of conservation of mass?
Answer 5051)What unit do we use for concentration?
Answer 5152) What is 1 mole of particles?
Answer 5253) What is the formula to calculate moles?
Answer 53